Powerful painkiller recalled after packaging mix-up

The nationwide quarantine was issued on Friday. Photo: AAP
A batch of popular painkillers has been pulled from pharmacy shelves after another medication that is four times stronger was found in one packet.
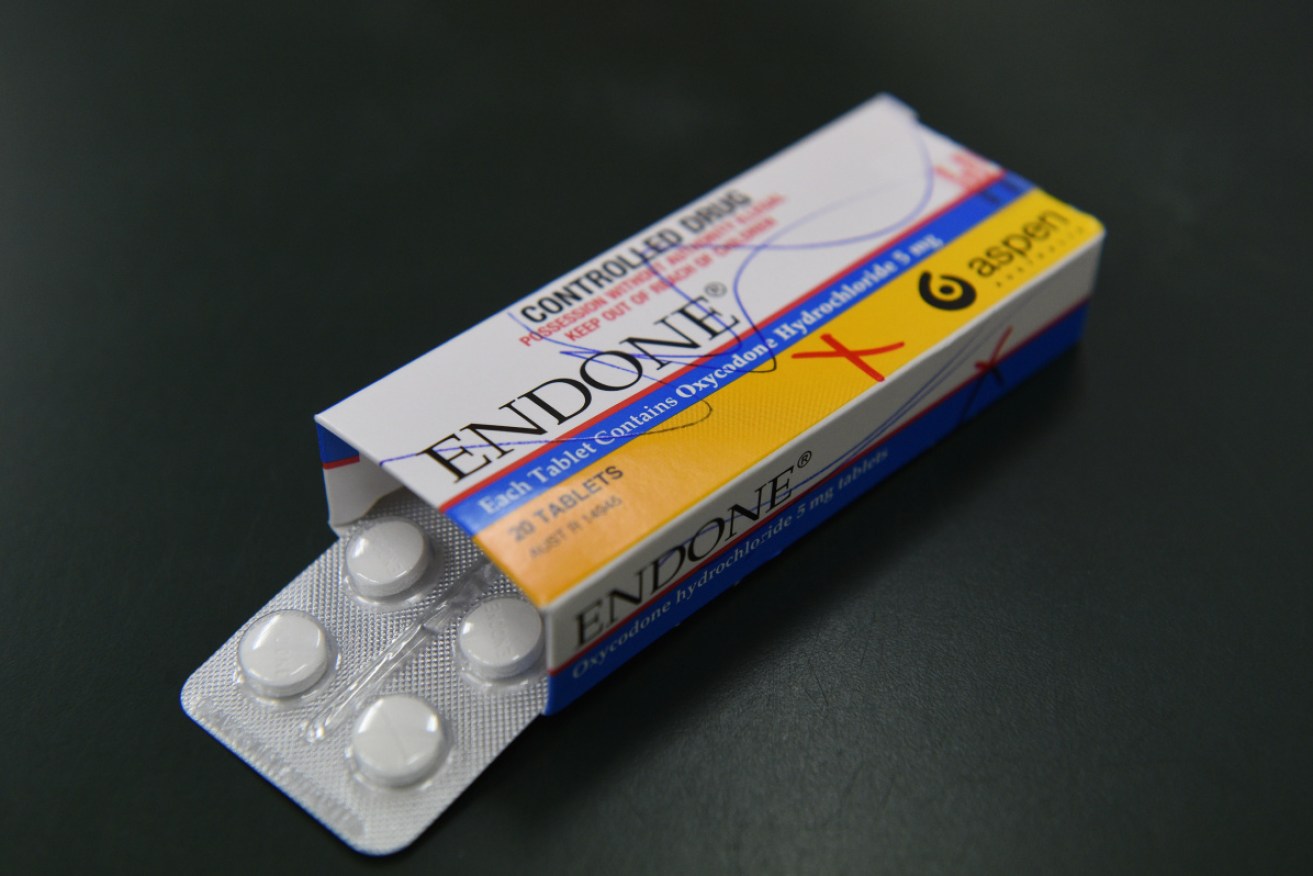
The nationwide recall was issued after a sleeve of the super-strong Anamorph, which contains morphine, was found inside a packet of five-milligram Aspen Pharma Endone in South Australia on July 26.
The Therapeutic Goods Administration, which issued the recall, said there was concerns over people accidentally overdosing.

A batch of popular painkiller medicine has been pulled from pharmacy shelves. Photo: TGA
“While the product names were correctly printed on the back of the blister sheet, the tablets and the blisters are a similar size, shape and colour, which increases the risk of inadvertently taking the wrong medicine,” they said.
“As a precautionary measure to mitigate against the risk of accidental overdose, the quarantine action for BN:CW612 was then escalated to a consumer level.”
Endone is a strong prescription pain medication containing oxycodone, while Anamorph contains morphine and is prescribed to treat severe pain when other medication or measures fail to provide relief.
Anamorph is usually given only to patients for a short amount of time. Side effects include drowsiness, nausea, seizures and comas.
It can also cause more serious health conditions and result in overdose if taken inadvertently.
Tweet from @TGAgovau
The recall applies to a single batch of the Endone, but it is not known how many packets remain on the market. The affected batch is number CW612, with an expiry date of November 2020.
Patients and pharmacists should check packets before taking the medication.
“Before taking any tablets from the affected batch of Endone five-milligram, patients should visually inspect blister sheets to ensure they contain the correct medicine,” the TGA said.
“As a further precautionary measure, pharmacists are also being asked to visually inspect the contents of packets of Endone five-milligram tablets from the affected batch to ensure they are correct before dispensing.”