‘Historic moment’: Drug slows cognitive decline in Alzheimer’s


The new drug won't put a demented brain together again, but may help to enjoy some normalcy for longer. Photo: Getty
It’s being widely reported – and celebrated by experts – that an experimental drug has, for the first time, “unambiguously” slowed the cognitive decline and brain damage in Alzheimer’s patients.
All we really know comes from a drug company press release, and full data is expected to be released at a conference in November – but already esteemed scientists not involved in the trial are calling the results “historic”.
Rob Howard, Professor of Old Age Psychiatry, University College London, summed up the mood when writing: “God knows, we’ve waited long enough for this”.
The trial
The Phase III trial involved 1795 volunteers in the early stages of Alzheimer’s disease who were given an infusion of lecanemab every two weeks. Their memory and cognitive function was regularly tested over 18 months.
The drug – developed by Tokyo pharmaceutical company Eisai, and biotechnology firm Biogen in Cambridge, Massachusetts – is a monoclonal antibody designed to clear clumps of amyloid proteins that are thought to kill brain cells and result in memory loss.

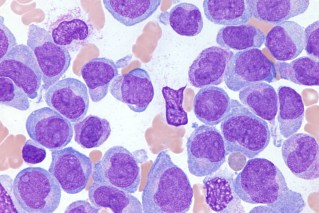
An Alzheimer’s brain showing amyloid protein clumps (the dark circles).
Over the course of the 18-month trial, the rate of cognitive decline in the participants had been slowed by 27 per cent, compared with a control group given a placebo.
These clinical benefits were small but significant – and for Alzheimer’s patients, any benefit will be latched onto.
For an explainer on what the results mean, see here.
The ‘amyloid theory’
We’ve all read about these amyloid proteins that gum up the brain, and most people probably take for granted that they are the root cause of the progressive brain death and dementia – and that might be the case.
But it remains a theory.
Developer Eisai has gone so far as to say the trial “proves” the theory is correct.
It’s too early to make such a claim.
So many drug trials have tried and failed to halt brain function decline by targeting amyloid proteins in dementia patients – and they all failed.
One exciting result does not a theory prove.
Side effects
During the trial, as reported by Nature, about 20 per cent (one in five) of participants who received lecanemab showed abnormalities on their brain scans that indicated swelling or a small amount of bleeding, “although less than 3 per cent of those in the treatment group experienced symptoms of these side effects”.
However, 9 per cent of those in the placebo group also suffered these side effects.
If the drug is given regulatory approval, and the developers are hoping to see this fast-tracked in the US, Europe and Japan by March in 2023, regular safety scans may be deemed necessary.
And some awkward history
Biogen, the Massachusetts-based developer, in June last year gained controversial approval from the FDA for the drug aducanumab, another monoclonal antibody designed to treat Alzheimer’s – but there was no proven clinical benefit.
As I reported at the time, in one of two clinical trials, the drug, aducanumab, was found to slow the rate of cognitive decline by about 25 per cent in patients with mild cognitive impairment (MCI), a precursor to dementia, or very early Alzheimer’s dementia.
Professor Chris Rowe – a nuclear medicine physician and neurologist – told me that the treatment should have been restricted to these patients.
But in the US, aducanumab was made available to anyone who could afford it.
Professor Rowe said MCI means “you have memory problems, and don’t do too well on testing, but you’re coping with life pretty well”.
He said that the drug – developed over a fraught decade – might give patients with severe dementia a couple of extra months of living. But they would still be unable to feed or wash themselves, the damage to their minds profound and irreversible.
Aducanumab would be of no use to them.
So the big question might be: is this an historic moment or history repeating itself?
For the moment, excitement runs high.
What the experts say
Professor Tara Spires-Jones, Group Leader at the UK Dementia Research Institute at the University of Edinburgh, said:

Professor Tara Spires-Jones
“Since the data is not yet available to the scientific community, I can’t comment yet on how robust the findings are. If the data holds up to scrutiny, this is indeed fantastic news.
“While this is not a ‘cure’ in that it doesn’t bring people back to normal, slowing cognitive decline and preserving the ability to perform normal daily activities would still be a huge win because people could live well for longer with Alzheimer’s disease.”
Dr Charles Marshall, Clinical Senior Lecturer and Honorary Consultant Neurologist, Wolfson Institute of Population Health at Queen Mary University of London, said:
“The headline results suggest that this is the first treatment that unequivocally slows down the progression of Alzheimer’s disease. This is very exciting because it represents the first compelling evidence that Alzheimer’s disease can be successfully treated, and in particular that removing abnormal amyloid protein from the brain is worth pursuing further as a treatment strategy.”

Professor Masud Husain
Professor Masud Husain, Professor of Neurology, University of Oxford, said:
“While the summary of the results certainly seems very encouraging, we have to be cautious until we are allowed to review the data fully. It is also important to bear in mind that the trial results apply only to people with mild Alzheimer’s disease, not everyone with the condition, and that there were important side effects of the drug, including bleeds in the brain.”
Professor Bart De Strooper, Director of the UK Dementia Research Institute, said:
“This is also a very encouraging result for the Alzheimer’s research community, demonstrating that we are moving in the right direction by targeting clearance of the amyloid beta protein. There is real momentum in the field and several exciting trial results on the horizon in the coming year.
“It’s important that we aim to administer lecanemab, and drugs like it, as early as possible in the disease course. Changes to the brain begin decades before symptoms appear in Alzheimer’s. Therefore, intervening rapidly gives these medicines the best chance of slowing brain degeneration before too much damage is done, and positively impacting the lives of those affected.”

Professor Rob Howard
Professor Rob Howard, Professor of Old Age Psychiatry, UCL, said:
“This is an unambiguously statistically positive result and represents something of an historic moment when we see the first convincing modification of Alzheimer’s disease. God knows, we’ve waited long enough for this.
“Important now that Eisai move quickly to publish the full data, including sensitivity analyses to look at the effects of functional unblinding in those participants who developed ARIA side-effects.”
The above comments have been edited for length. To read more comments from experts in full see here.